EBV Serology and Clinical Interest
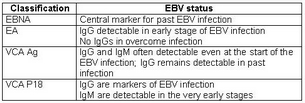
Serology is used to confirm EBV infection and involves interpreting the profile of the most relevant immunological responses : viral capside antigens (VCA) IgG and IgM, and Epstein-Barr Nuclear Antigens (EBNA) IgG.
VIDAS® EBV – Why 4 markers in 3 tests?
Early Antigen (EA) combined with VCA P18 included in VIDAS VCA/EA IgG is an aid to improving the global positive response of the kit used to confirm primary stage EBV infection as EA IgG are present in 80% of infectious mononucleosis cases.
VIDAS® EBV - 3 Tests for a Confident Diagnosis
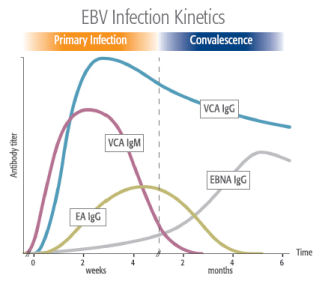
- Our 3 tests have been designed to provide the most relevant EBV antibody responses when used in combination for staging EBV infection.
- 3 bioMérieux peptide patents (EA P54, VCA P18, EBNA P72)
 |
|
VIDAS VCA IgM
Ref. 30237 - 30 tests |
| |
|
| |
VIDAS VCA/EA IgG
Ref. 30236 - 30 tests |
| |
|
| |
VIDAS EBNA IgG
Ref. 30235 - 30 tests |