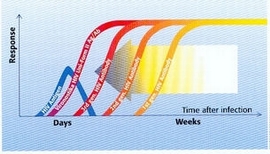
With HIV antibody tests becoming ever more sensitive, the so-called window phase, i.e. the time between infection and detection of HIV antibody, has been reduced. Further shortening of that window phase can be achieved by incorporation of HIV antigen detection in such highly sensitive HIV antibody tests. With the combined detection of HIV antigen and HIV antibody in one single test the 4th generation of HIV screening tests has come available.
The Vironostika HIV Uni-Form II Ag/Ab test, with ist pearl shaped, freeze-dried conjugate present in each well, is a 4th generation HIV assay setting new standards in sensitivity for the immunological detection of HIV-1, HIV-1 Group O and HIV-2 infection. It is based on the Uni-Form II concept with its ultimate user-friendliness.
Uni-Form II: the pearl that puts Elisa a step ahead.